
For example, halogen-bonded complexes of I 2 and Br 2 were central to the development of Mulliken’s theory of charge–transfer interactions. In particular, studies of the interactions of molecular halogens X 2 with Lewis bases played important roles in advancing understanding of structure and bonding.
Although this interaction is a relative newcomer to the supramolecular chemistry arena, it has a long history. Halogen bonding (XB) occurs when there is an attractive interaction between a Lewis basic site (the halogen bond acceptor) and an electron-deficient, covalently bonded halogen (the halogen bond donor Fig. 1).
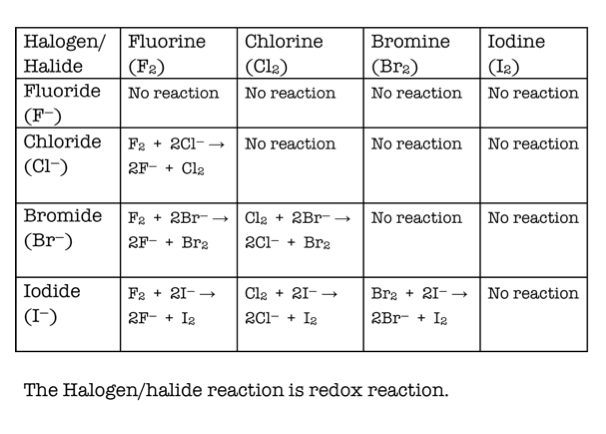
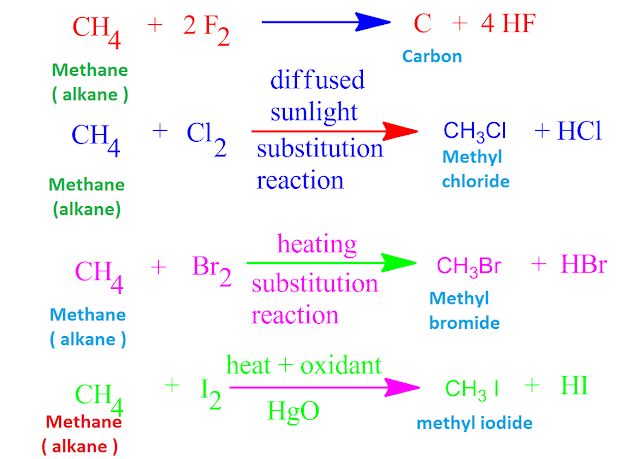
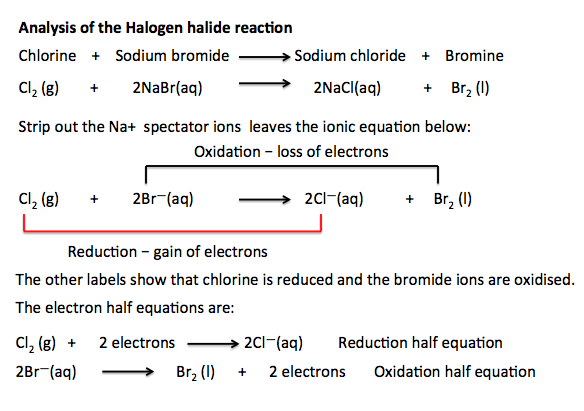
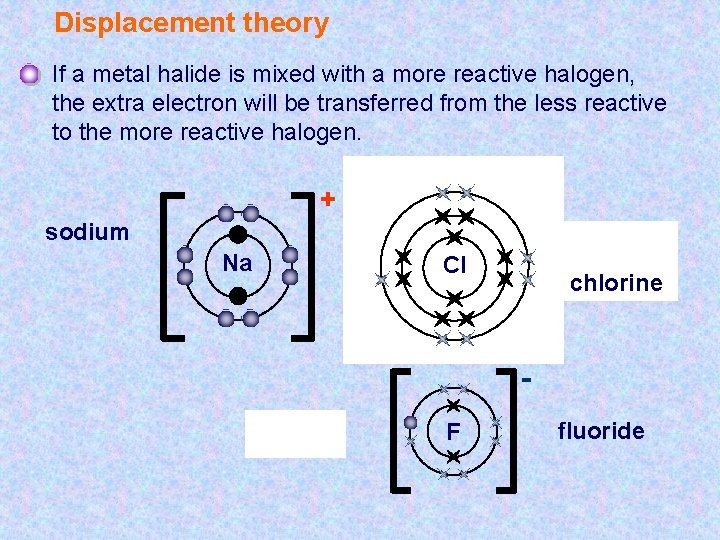
Halogens reactivity how to#
Therefore, this chapter will focus only on the challenge of how to replace halogen flame retardants by halogen-free flame retardants and some successful examples will be presented. Owing to the numerous available chemicals and polymer systems, it is impossible to present all their different combinations in a chapter. Continuing pressures regarding environmental and toxicity issues have generated demands for some halogen-containing flame retardants to be replaced by halogen-free flame retardants. However, some halogenated flame retardants have been claimed to be a source of toxic halogenated dibenzodioxins and dibenzofurans, which have more recently greatly limited their wide usage. Halogen-containing flame retardants, including about 50–100 kinds of halogen-containing compounds that cover most of the market requirements, are one of the most commonly adopted flame retardant groups due to their highly effective flame retardancy and low price. Wang, in Advances in Fire Retardant Materials, 2008 4.1 Introduction An overview of the current state of halogen bonding of halogen(I) ion complexes is included, as well as the many advancements made to those fields in recent years. Halogen(I) complexes form a special family manifesting a strong halogen bond, which demonstrate good stability in both the solution and solid-states. A halogen(I) ion itself is highly reactive, but can be ‘trapped’ in between two Lewis bases creating a + 3-center, 4-electron halogen bond between the halogen(I) ion and two donor atoms of the Lewis bases (typically nitrogen). When an electron is completely removed from a halogen, a halogen (I) ion (X +) is formed. This σ-hole then interacts with electron-rich nucleophiles (such as Lewis bases, B) forming a linear R–X The polarization, caused by an electron withdrawing group (R), induces electron deficiency onto the halogen atom, creating an electropositive region known as a σ-hole.
Halogen bonding occurs between a positive region of a polarized halogen (X), most commonly with an iodine or bromine atom. Kari Rissanen, in Reference Module in Chemistry, Molecular Sciences and Chemical Engineering, 2022 Abstract The halogen oxides are the anhydrides of acids. The hydrogen halides are all acidic although HF is a weak acid. Both anions and cations of interhalogens are known, usually formed by removing or adding X − to an interhalogen molecule. Most are extremely reactive and behave as fluorinating agents. Not all of the possible compounds are known and only iodine forms IF 7. Halogens form compounds containing two different halogens, the interhalogens, having the formulas XX′ n where X is the less electronegative element and n = 1, 3, 5, or 7. Fluorine and chlorine are produced electrochemically. Bromine and iodine are found in seawater and as minor constituents in chlorides. Fluorine occurs in fluorite, CaF 2, and other minerals.
The elements are found in nature with salt, NaCl, being the most abundant compound of chlorine. All are oxidizing agents with fluorine being extremely strong. The halogens are reactive elements that consist of diatomic molecules. House, in Descriptive Inorganic Chemistry (Third Edition), 2016 Abstract


 0 kommentar(er)
0 kommentar(er)
